PatientView’s Corporate Reputation of Pharma 2023/24: Insights from Specialised Cancer Patient Groups
July 2024:
The field of oncology, cancer research and cancer treatment has seen continued investment over the last few years. In the UK, cancer incidence rates are projected to rise by 2% [1] between 2023-2025 and 2038-2040, to 625 cases per 100,000 people [2] on average each year by 2038-2040. In the United States, overall cancer mortality rate has been steadily decreasing since the late 1990s [3] – but some forms of cancer have either remained at the same mortality rate or even increased.
The need for different approaches for different forms of the disease is clear, as “cancer” is becoming a less-effective umbrella term for a variety of different conditions and diseases. Cancer treatments in 2024 are diversifying to match this growth of the oncological field of study. Modern tools like AI prediction aids are being developed to help with immunotherapy treatment plans [4] and, in the US, national-level initiatives are being launched to test the effectiveness of drug combinations that target specific cancer mutations.[5]
In June 2024, PatientView released its annual report on the corporate reputation of the pharmaceutical industry as perceived by cancer patient groups. Based on a survey conducted from November 2023 to late February 2024, PatientView gathered responses from 570 cancer patient groups, including those focusing on blood, breast, lung, and prostate cancers. The survey specifically aims to gauge the opinions of cancer patient groups regarding the pharmaceutical industry’s reputation, particularly in developing and delivering new cancer treatments.
In the 2023/24 edition of the report, PatientView looked at the differing opinions of respondents, which focused on four different types of cancer: 77 blood cancer groups, 126 breast cancer groups, 39 lung cancer groups, and 36 prostate cancer groups.
This blog highlights the key findings and major differences of opinion between these four sets of cancer patient group respondents, and their recommendations for improving the pharma industry’s relationship with patients.
Industry-wide results
The report indicated a generally positive view of the pharmaceutical industry’s innovation efforts, with new cancer treatments raising the hopes and expectations of patient groups. However, several barriers hinder patient access to these innovations:
- Length of Clinical Trials: Prolonged clinical trials delay the availability of new treatments.
- Manufacturing Challenges: The industry’s ability to scale automated manufacturing efficiently remains limited.
- Drug Pricing: High costs of new treatments pose significant challenges for healthcare systems and patients, particularly in countries with limited resources or where patients bear the costs directly.
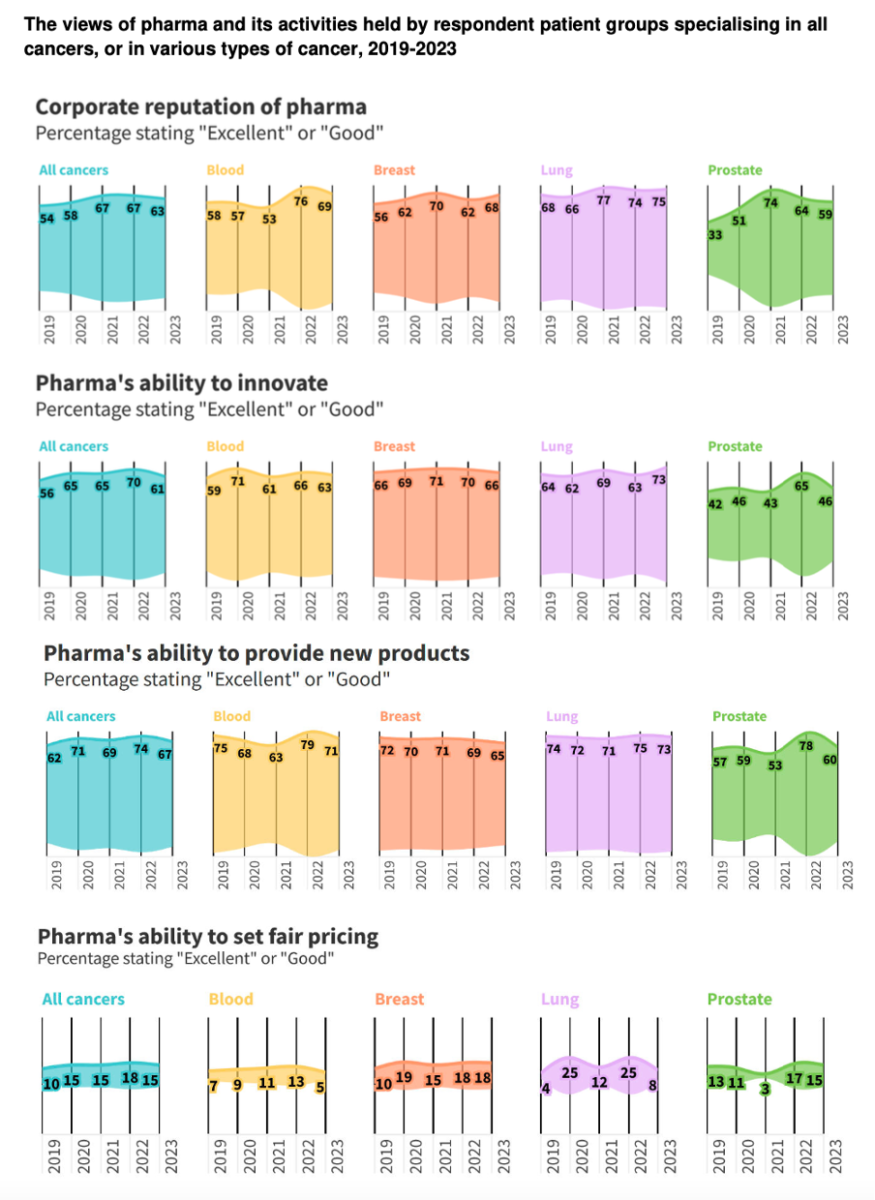
Specific feedback from cancer patient groups
Blood Cancer Groups
Respondents from blood cancer groups highlighted the prohibitive costs of innovative treatments, making them inaccessible in many countries. They called for greater collaboration between pharmaceutical companies, national governments, and the EU to ensure fair access to treatments.
“The price of the new individual innovative treatments, which makes it difficult to get them approved in countries with a national health system—and difficult to pay for in countries where the patients themselves have to pay, or take out insurance.” —Blood cancer patient group, Denmark
Breast Cancer Groups
Breast cancer groups emphasized the need for reasonable pricing and inclusion of essential medicines in state healthcare provisions. They stressed the importance of making treatments available globally at fair prices.
“Medicines must have reasonable prices for access to them. The state institutions must also include them in the essential list of medicines, but not that they are in smaller quantities than the number of patients.” —Breast cancer patient group, Kosovo
Lung Cancer Groups
Lung cancer groups noted improvements in drug accessibility but called for increased transparency around compassionate-use programs to further enhance access.
“More transparency around compassionate-use programme access.” —Lung cancer patient group, UK
Prostate Cancer Groups
Prostate cancer groups pointed out a lack of focus on new treatments for their condition. They recommended more aggressive cooperation with insurers to secure coverage for new medications and better alignment with EU regulatory processes.
“Work aggressively with insurers to garner coverage for new medications, to benefit patients who could benefit most from the new drugs.” —Prostate cancer patient group, USA
Corporate Reputation – Cancer patient group rankings
The report also provides rankings of pharmaceutical companies based on their corporate reputation as judged by cancer patient groups familiar with or working with these companies. The companies featuring in the top rankings are shown below.
Top-ranked companies
- All Cancers: 1st: Roche, 2nd: AstraZeneca, 3rd: Pfizer
- Blood Cancer: 1st: Janssen, 2nd: Novartis, 3rd: Roche
- Breast Cancer: 1st: Roche, 2nd: Novartis, 3rd: AstraZeneca
- Lung Cancer: 1st: Roche, 2nd: AstraZeneca, 3rd: Pfizer
- Prostate Cancer: 1st: Janssen, 2nd: Bayer, 3rd: Pfizer
PatientView’s 2023/4 report provides critical insights into how cancer patient groups perceive the pharmaceutical industry’s efforts. Despite significant advancements in cancer treatment innovations, the industry faces challenges in ensuring broad and equitable access to these treatments. The feedback from patient groups underscores the need for the pharmaceutical industry to address pricing, regulatory, and manufacturing barriers to meet patient needs more effectively.
By aligning more closely with patient needs and perspectives, companies can improve their corporate reputation and contribute more effectively to global cancer care.
To obtain a copy of the full report, please contact Alex Wyke at PatientView via email ([email protected]).
- https://www.cancerdata.nhs.uk/incidence_and_mortality
2. https://www.cancerdata.nhs.uk/incidence_and_mortality
3. https://www.cancer.gov/about-cancer/understanding/statistics
4. https://www.cancer.gov/news-events/press-releases/2024/ai-tool-predicts-response-to-immunotherapy
5. https://www.cancer.gov/news-events/press-releases/2023/combomatch-precision-medicine-cancer-initiative
